Understanding LEL vs vol%: A Guide to Gas Concentration Measurements
Introduction
When it comes to measuring gas concentrations, particularly in safety applications, two common units often appear: LEL (Lower Explosive Limit) and vol% (volume percent). Understanding the difference between these measurements is crucial for anyone working with combustible gases or in hazardous environments.What is LEL?
LEL stands for Lower Explosive Limit, which represents the threshold
for how much flammable gas must be present in the air before an explosion or fire can occur. At
concentrations below the LEL, the mixture is too "lean" to burn. For example a very small amount of
propane in a room will not pose a fire hazard. Nothing will happen if we light a match or
create a spark in the room.
If the amount of propane increases, for example as a result of a gas leak, the atmosphere
will eventually become ignitable and can explode from a spark. LEL represents precisely the
point where the air becomes flammable.
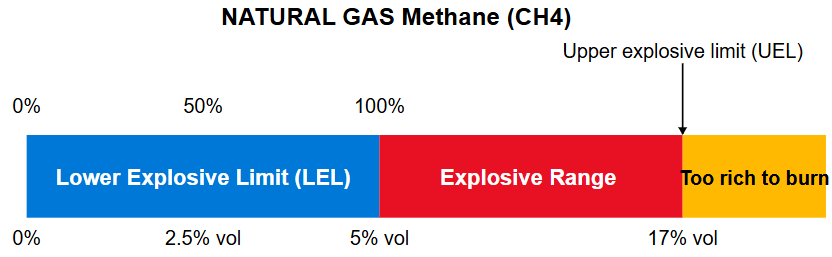
LEL is expressed as a percentage of the minimum concentration required for combustion.
For example, methane has an LEL of 5 vol%. This means that when methane makes
up 5% of the total volume of a methane-air mixture, it reaches its lower explosive limit.
What is UEL?
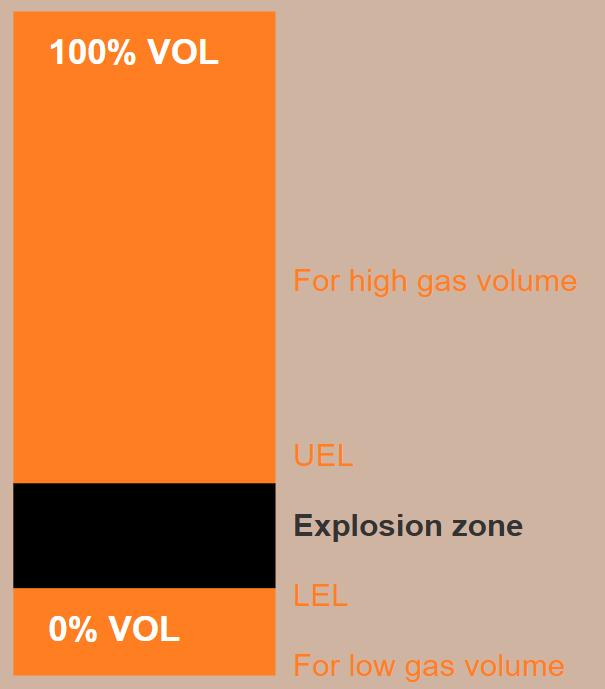
All combustible gases have an explosive range - an interval where the atmosphere is flammable. This range starts at LEL - Lower Explosive Limit, and ends with UEL - Upper Explosive Limit. If the air in a room contains 40% propane, the gas mixture is too "thick" to be ignited. We have exceeded the UEL, and the atmosphere is no longer ignitable because there is not enough oxygen present to support a fire. Both LEL and UEL vary from gas to gas. See table on the bottom for most common gases.
We can use two different combustible gases as examples:
Methane (CH4): The lower explosive limit for methane in air is around 5 volume percent. This is the ISO reference.
If the atmosphere contains less than 5 volume percent methane, the amount of gas is too small for the air to ignite.
When the methane content exceeds 5 volume percent, the fire hazard increases. The UEL for methane is around 15 volume percent.
We can therefore say that we have a flammable atmosphere if there is between 5-15 volume percent methane in the air.
Propane (C3H8): The LEL for propane in air is around 2.1 volume percent. The UEL for propane is around 9.5 volume percent.
A flammable atmosphere for propane at normal oxygen levels is therefore between 2.1-9.5 volume percent.
We can thus see that it takes less propane in a room to exceed the LEL than methane. There is great variation in the explosive range of different gases.
The LEL and UEL values should be used as general guidelines as pressure, temperature, and other substances in the air can have an impact on the values.
What is vol%?
Volume percent (vol%) is a straightforward measurement indicating the percentage of a specific gas in the total volume of a gas mixture. It's a direct ratio measurement—10 vol% means that 10% of the volume of the gas mixture consists of the target gas.
The Critical Difference
The key difference between LEL and vol% lies in what they reference:
- Vol% - measures the actual percentage of a gas in a mixture
- LEL% - measures the percentage of the way to reaching the explosive threshold
For example, if methane (CH4) has an LEL of 5 vol%, then:
- 5 vol% methane = 100% LEL (the mixture is at its lower explosive limit)
- 2.5 vol% methane = 50% LEL (the mixture is halfway to its explosive limit)
Why Use LEL Instead of vol%?
Safety professionals often prefer LEL because:
- Immediate Safety Context: 50% LEL instantly tells you you're halfway to a potentially dangerous situation, regardless of which gas is being measured.
- Standardized Alarms: Safety systems can use consistent alarm points (e.g., 10% LEL and 25% LEL) across different gases.
- Ease of Interpretation: Personnel don't need to memorize the explosive limit value for each gas they might encounter.
Conversion Between LEL and vol%
Converting between LEL and vol% is straightforward when you know a gas's LEL value:
Where "LEL value" is the vol% concentration at which the gas reaches 100% LEL.
For example, with methane (LEL value = 5 vol%):
50% LEL =(50x5) /100 = 2.5 Vol%
Conclusion
While vol% gives you the absolute concentration of a gas, LEL provides crucial context about safety margins. For anyone working with combustible gases, understanding both measurements—and how they relate to each other—is essential for maintaining a safe working environment.
| Gas | Lower Explosive Limit (LEL) in VOL% | Upper Explosive Limit (UEL) in VOL% |
|---|---|---|
| Methane | 4.4 | 17.0 |
| Propane | 1.7 | 10.9 |
| Acetylene | 2.3 | 100.0 |
| Carbon Monoxide | 10.9 | 74.0 |
| Heptane | 0.85 | 6.7 |
| Gasoline | 1.4 | 7.6 |
| Hydrogen Sulfide | 4.0 | 45.5 |
| Acetone | 2.5 | 14.3 (100 °C) |
| Ammonia | 15.0 | 33.6 |
| Benzene | 1.2 | 8.6 |
| Butane | 1.4 | 9.3 |
| Ethylene | 2.3 | 36.0 |
| Toluene | 1.0 | 7.8 |
| Cyclohexane | 1.0 | 8.0 |
| Hexane | 1.0 | 8.9 |
| Hydrogen | 4.0 | 77.0 |
| Gas | Lower Explosive Limit (LEL) in VOL% | Upper Explosive Limit (UEL) in VOL% |
|---|---|---|
| Methane | 5.0 | 15.0 |
| Propane | 2.1 | 9.5 |
| Acetylene | 2.5 | 82.0 |
| Carbon Monoxide | 12.5 | 74.0 |
| Hydrogen | 4.0 | 75.0 |
| Ammonia | 15.0 | 28.0 |
| Butane | 1.8 | 8.4 |
| Ethylene | 2.7 | 36.0 |
| Benzene | 1.2 | 7.8 |
| Hexane | 1.1 | 7.5 |
| Acetone | 2.5 | 13.0 |
| Hydrogen Sulfide | 4.3 | 45.0 |
| Ethane | 3.0 | 12.5 |
| Ethanol | 3.3 | 19.0 |
| Methanol | 6.0 | 36.0 |
| Pentane | 1.4 | 8.0 |
